Project Description
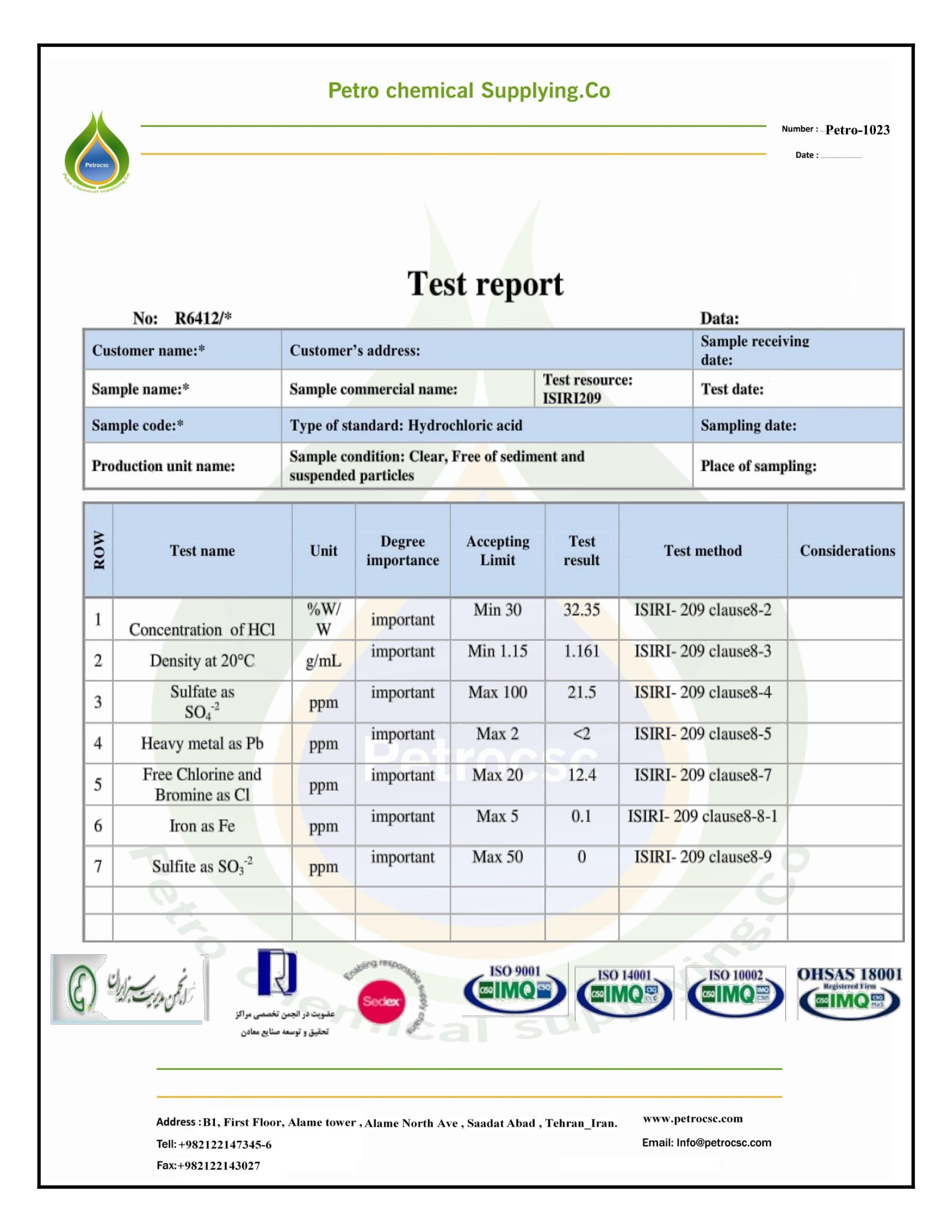
Hydrochloric Acid
Manufacturing
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride gas. Hydrochloric acid is produced in the United States primarily by four basic methods: the chlorination of organic chemicals; the combination of hydrogen and chlorine; the salt-sulfuric acid production process; and, as a co-product in the manufacture of silica. Most hydrochloric acid is produced from the chlorination of organic chemicals with much smaller amounts from the other processes.
FEATURES
Uses
Hydrochloric acid is an important and widely used chemical. The largest end uses for hydrochloric acid are steel pickling, oil well acidizing, food manufacturing, producing calcium chloride, and ore processing.
Steel Pickling
Hydrochloric acid is used in pickling operations for carbon, alloy and stainless steels. Steel pickling is the process by which iron oxides and scale are removed from the surface of steel by converting the oxides to soluble compounds. Pickling is required for steel products that undergo further processing such as wire production, coating of sheet and strip, and tin mill products. Hydrochloric acid is used primarily for continuous pickling operations in which hot-rolled strip steel is passed through a countercurrent flow of acid solution.
In addition to steel pickling, hydrochloric acid is used in aluminum etching, metal prefixing for galvanizing and soldering, and metal cleaning.
Oil Well Acidizing
Hydrochloric acid is used both to remove rust, scale and undesirable carbonate deposits in oil wells to encourage the flow of crude oil or gas to the well. This use is called “stimulation”. Acidizing is generally done in carbonate or limestone formations by stimulation. An acid solution is injected into the formation, which dissolves a portion of the rock and creates a large pore structure in the formation, increasing its effective permeability and the flow of oil.
Food
The food industry uses hydrochloric acid in the processing of a variety of products. A major use of hydrochloric acid by the food industry is for the production of corn syrups such as high-fructose corn syrup (HFCS).
Much of the hydrochloric acid consumed in the HFCS industry is used to regenerate the ion exchange resins that are employed to remove impurities. Hydrochloric acid can also be used to acid-modify cornstarch and to adjust the pH of intermediates, final product and wastewater. The largest use of HFCS is in the production of soft drinks, which accounts for 70-75% of demand.
Hydrochloric acid is also used in other food processing applications including the production of hydrolyzed vegetable protein and soy sauce. It is used in acidulating crushed bones for the manufacture of gelatin and as an acidifier for products such as sauces, vegetable juices and canned goods.
Hydrochloric acid is consumed in the production of artificial sweeteners. It is consumed in the production of lysine, choline chloride (both used primarily as animal feed additives) and citric acid.
Production of Calcium Chloride
Neutralizing hydrochloric acid with limestone (CaCO3) produces calcium chloride. The largest use for calcium chloride is highway deicing with production dependent on weather conditions. Other uses include dust control, industrial processing, oil recovery, concrete treatment and tire ballasting. Calcium chloride is also used in oil recovery products such as drilling muds and work-over/completion fluids.
Other
Aqueous hydrochloric acid is used in a variety of miscellaneous applications. These include recovery of semiprecious metals from used catalysts, use as a catalyst in synthesis, use in catalyst regeneration, pH control, regeneration of ion exchange resins used in wastewater treatment and electric utilities, neutralization of alkaline products or waste materials, and in brine acidification for use in the production of chlorine and caustic soda.
Hydrochloric acid is also used in many other production processes for organic chemicals. It can be used in the production of p-phenylenediamine, polycarbonate resins, bisphenol A, polyvinyl chloride resins, and ethanol (from ethylene).
The pharmaceutical industry consumes hydrochloric acid as a catalyst in synthesis, for pH control, for deionization of water and as a reduction agent (e.g., in the production of ascorbic acid and para-aminobenzoic acid).
Numerous other uses of hydrochloric acid include the manufacture of dyes and pigments; the removal of sludge and scale from industrial equipment; the deliming, tanning and dying of hides by the leather industry; manufacture of permanent wave lotion; the carbonizing of wool; use as a bleaching and dyeing assistant in the textile industry; and the purification of sand and clay.
Click on the link to order the caustic soda flake.
SPECIFICATION